Cosmetic Ingredients and Product Approval in China
Cosmetic Supervision and Administration Regulation (CSAR)
Overview
The China Cosmetic Supervision and Administration Regulation (CSAR) is the main regulation in charge of overseeing cosmetic ingredients and products in China. It was issued by the China National Medical Products Administration (NMPA) in 2020 and is accompanied by other rules such as the Technical Guidance for Submission of Cosmetic Ingredient Safety Information and the Safety and Technical Standards of Cosmetic Ingredients.
Obligations on Cosmetic Ingredients
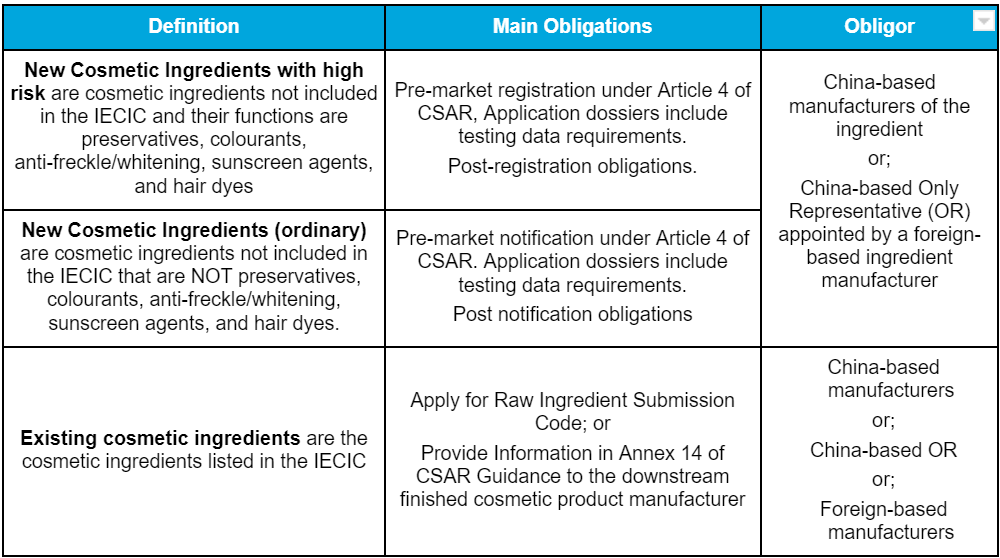
CSAR regulates new and existing cosmetic ingredients and also the use of certain ingredients in cosmetic finished products. The types of cosmetic ingredients and obligations are summarised in the following table.
Please note: Manufacturers of existing ingredients shall fulfil the obligations (Ingredient Submission Code or Annex 14) and communicate the information with their downstream manufacturers of finished cosmetic products before the following deadlines.
Scenario 1_Fulfil the obligation before placing on the market: If the existing ingredient is supplied to manufacture a cosmetic finished product that will be registered or notified after 1st Jan 2024.
Scenario 2_Fulfil the obligation before 1st Jan 2024: If the existing ingredient is listed in the Safety and Technical Standards of Cosmetic Ingredients.
Scenario 3_Fulfil the obligation before 1st Jan 2024: If the existing ingredients are of high risk (preservatives, colourants, anti-freckle/whitening, sunscreen agents, and hair dyes) supplied to China to manufacture a cosmetic finished product that has been registered or notified from 1st May 2021 to 1st Jan 2024.
Obligations on Cosmetic Products
Article 16 of CSAR specifies the types of cosmetic products and their obligations:
Our Services/Solutions
Yordas can support our client in fulfilling the obligations under CSAR, including
Existing cosmetic ingredient code application, Annex 14 preparation and translation;
New cosmetic ingredients registration/notification;
Cosmetic product registration/notification;
Only Representatives (responsible person) appointment.
Contact our team today so we can help you execute your compliance obligations. Sign up for our monthly newsletter for all of the latest regulatory news and the most important industry updates.